Scientists have reversed advanced heart failure in animals using gene therapy, giving hope to what many believe will happen soon: living 120 to 150 productive years, writes Satyen K. Bordoloi.
Ray Kurzweil, the world’s most renowned futurist, has made accurate predictions about technology more times than most. He foresaw the triumph of computers over humans in chess, the exponential increase in computational power inversely proportional to cost, and the global exchange of information via what we now know as the internet. Among his many predictions, two are yet to materialize: singularity by 2045 and immortality by 2030. At 76, Kurzweil is keeping himself in peak health, ready for 2030.

The prospect of immortality by 2030 seems unlikely. Despite numerous advancements, a key frontier, the most crucial hurdle in the longevity puzzle, remains elusive: preserving the human heart and ensuring its longevity. This is not just important for extending life but also for enhancing the quality of life for everyone in general.
When we learn of someone’s death, the first question we ask is: how? For those with known ailments, the answer is straightforward. However, there are those who maintain good health, do not suffer from any debilitating conditions, and die outside of a hospital. For such individuals, we often say they died of ‘old age’. This, as any doctor will tell you, is a euphemism for heart failure. Even if other organs remain healthy, the heart eventually weakens, ceasing to beat one day, resulting in the death of the individual.
The human heart, which tirelessly maintains our life’s rhythms for decades, sometimes over a century, is both resilient and fragile. When it falters, its consequences ripple through our lives. The biggest enemy of the heart, and the reason why otherwise healthy elderly people die, is advanced heart failure. The heart is our most valuable asset, and its failure is a formidable adversary that has long defied conventional treatments. But what if we could alter the very script that causes the heart to slow down with age? To do that, we would need to modify the script’s location, i.e., our DNA. Unsurprisingly, gene therapy has emerged as a beacon of hope, illuminating the darkness of cardiac dysfunction.
In a recent study published in the journal Cardiovascular Research, researchers used gene therapy to treat advanced heart failure and improve cardiac function in animals. This proved to be a lifeline for a heart on the brink of failure and has the potential to not only transform individual lives but also reshape the world as we know it.
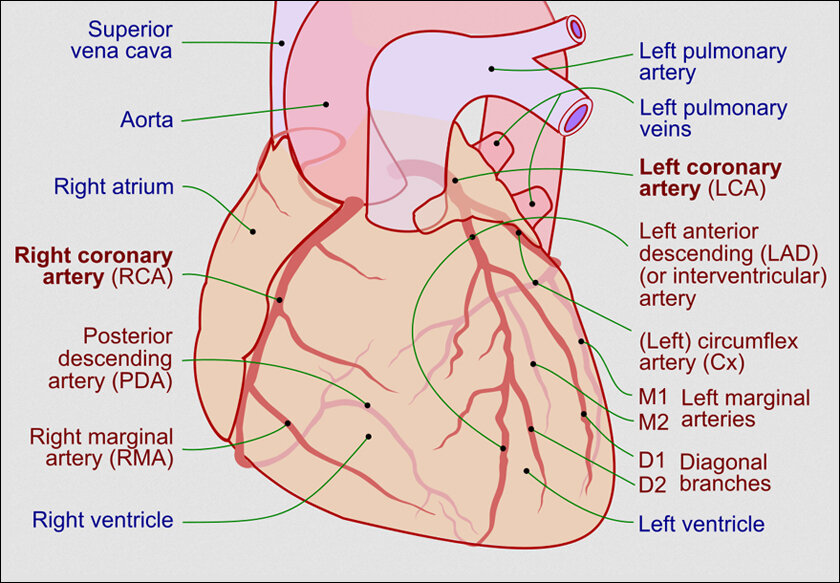
HOW DOES THE HEART FAIL:
Imagine your heart as a bustling city, its arteries and veins the highways carrying vital supplies – oxygen and nutrients – to its hardworking residents, the heart muscle cells. When a crucial highway gets blocked by a landslide of plaque build-up, it leads to a traffic jam, i.e., a heart attack. This blockage starves the heart cells of oxygen, causing them to die and leave behind empty lots. After a heart attack, most of the lost cells cannot rejuvenate. Nature attempts a patch job, filling the gaps with collagenous scar tissue formed by fibroblasts. These stabilize the damage but lack the pumping prowess of the original muscle cells.
Thus, if multiple heart attacks or damage occur, each leaves behind a mark. The once efficient city becomes riddled with scar tissues, its expansion and contraction capabilities drastically reduced. This progressive stiffening and remodeling of the heart, known as cardiac remodeling, leads to cardiac failure as the heart struggles to pump blood against the resistance of scar tissues. The body’s tissues and organs, starved of vital nutrients, begin to falter, leading to a range of debilitating symptoms and potentially life-threatening complications.

(Image Credit: Lexica Art)
HOW DOES GENE THERAPY REVERSE THIS:
Currently, it is not possible to stimulate a heart that has entered cardiac failure or to generate new heart cells to repair it. However, Dr. Tamer M. A. Mohamed, corresponding author of the study, associate professor of surgery and medicine, and director of cardiac regeneration at Baylor College of Medicine, Houston, Texas, and his colleagues have been working on this problem for years. In a previous study, he and his collaborators successfully used gene therapy to improve acute cardiac dysfunction in animals. This method effectively delivered genes with the sole intention of promoting the proliferation of heart cells, thus creating new heart muscles. The strengthened heart’s ability to keep blood flowing improved and prevented congestion in the kidneys and lungs of the rats and pigs it was tested on.
The current test is an expansion of the same experiment. But this time, instead of using the same gene therapy during acute heart failure and early heart disease, they tried it late during heart disease, i.e., stage four, where the heart has been severely damaged. Four months after the treatment, the first author of the study, Dr. Riham R E Abouleisa, assistant professor of surgery-cardiothoracic surgery at the same institute, was surprised to find evidence of cell growth in the heart and a reduction in the size of scar tissue, which led to the improvement of cardiac function. Although heart and lung congestion did not improve, liver and kidney functionality did.
As Dr. Riham mentioned in the paper, “The findings show for the first time that contrary to expectations, it is possible to induce heart cell proliferation during advanced states of heart failure and improve heart function, with some beneficial effects on the liver and kidneys’ functions.” Currently, there are no treatments to improve the conditions of patients during advanced heart failure. However, this study shows that it is indeed possible, and this approach offers the potential to develop future new therapies for this and similar heart diseases.
This means that it is never too late to repair the most important organ in our body. And if we manage to do that, the life expectancy of patients could be dramatically increased in the future. What this also does is improve the quality of life, as currently, the only option for patients like this is palliative care and waiting to die.

This is not the only study out there. Different studies conducted worldwide are trying to improve the heart in various ways. Some are trying to halt the natural degeneration process, while others, like this one, are striving to improve the heart’s functionality even when it has degenerated. One of the most promising ones uses stem cells. Plucked from bone marrow or cultivated in labs, they could soon, when inserted into the heart, stitch it in its own fabric, keeping scar tissues at bay.
Put together, research on heart diseases seems to be heading in the right direction, and common ailments of the heart will be a thing of the past. This will not only ensure that we live longer lives, but also that as long as we live, we are healthy and functional.

(Image Credit: Lexica Art)
Kurzweil envisioned a future where tiny robots running through our blood will repair our bodies at the cellular level, making us immune to diseases, ageing, and ultimately death. What is instead happening is something much simpler: cellular regeneration of organs is being conducted via different methods, including gene therapy. If nanotechnology reaches that level, it will be the icing on the cake.
We stand on the precipice of a new era in medical science. The heart, once considered an insurmountable challenge, is slowly revealing its secrets, and we are learning to mend it in ways we never thought possible. As we continue to unlock these mysteries, we inch closer to a future where the rhythm of life plays on, uninterrupted, for many more years than we ever dared to dream. 120 years of healthy life tomorrow; 150 to 200 in the not-so-distant future.
In case you missed:
- A Howl Heard Worldwide: Scientific Debate Roars Over an Extinct Wolf’s Return
- Tears of War: Science says women’s crying disarm aggressive men
- AI vs. Metaverse & Crypto: Has AI hype lived up to expectations
- AI Taken for Granted: Has the World Reached the Point of AI Fatigue?
- And Then There Were None: The Case of Vanishing Mobile SD Card Slots
- Copy Of A Copy: Content Generated By AI, Threat To AI Itself
- Quantum Leaps in Science: AI as the Assembly Line of Discovery
- Generative AI With Memory: Boosting Personal Assistants & Advertising
- India’s Upcoming Storm of AI Nudes & Inspiring Story Of A Teen Warrior
- Project Stargate: Dubious Origins in the 1970s to AI Goldrush in 2025









